The New York Academy Of Sciences
June 17, 2025
Astrology is prospering from times immemorial, taking part in on the hopes and anxiousness of mankind to find out about tomorrow. As an alternative, supernatural explanations should be left a matter of private belief exterior the scope of science Methodological naturalism maintains that correct science requires strict adherence to empirical study and impartial verification as a course of for correctly growing and evaluating explanations for observable phenomena.
Our persons are pushed by curiosity to increase fundamental knowledge and to look beyond the borders of their own discipline; their aim is to benefit science, and to make a contribution to addressing the main societal challenges of the future. Andrew is fascinated by cosmology — his favourite book is A Transient Historical past of Time by Stephen Hawking — however he sees Buddhism as answering different kinds of inquiries to science.
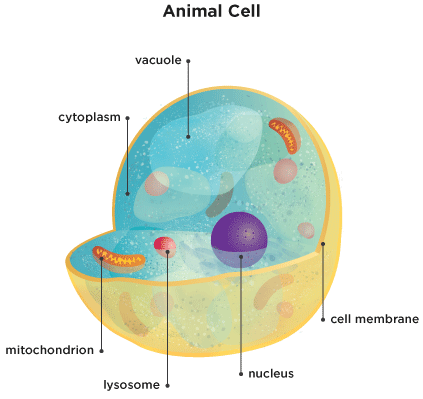
In a science camp, children would be able to be taught basic concept of heavy and lightweight via STEM activities. Youngsters be taught the aim of each body system, what the main organs of the system are and their specific features, and the way the organs work together to ensure that the system and body to function as a complete.
The …